Prostaglandins, powerful compounds first studied for their significant biological effects in medicine, have sparked considerable controversy in the beauty industry. Initially praised for their potential to enhance eyelash growth, these substances have also raised numerous health and ethical concerns.
The clean beauty movement, which emphasizes safety, transparency, and ethical practices, faces a particular dilemma with the inclusion of prostaglandins in cosmetic products.
In this article, we will explore the troubled history of prostaglandins in the cosmetics industry, focusing on:
- Their discovery and medical controversies: How prostaglandins were initially used in medicine and the issues that arose.
- Adoption in cosmetics: The risks that were overlooked during their introduction to beauty products.
- Regulatory battles: The struggles with approval and safety classifications.
- Ethical concerns: The impact on animal testing and the environment, and the resulting backlash from clean beauty advocates.
Table of Contents - Click to Go
The Discovery and Medical Controversies of Prostaglandin Synthesis

Prostaglandins were discovered by Swedish physiologist Ulf von Euler in the 1930s. These compounds, found throughout the human body, are involved in various essential processes, such as:
- Inflammation: Prostaglandins play a key role in the body’s inflammatory response.
- Blood flow: They help regulate blood flow to different tissues.
- Blood clot formation: Prostaglandins assist in the formation of blood clots, which are crucial for healing injuries.
Arachidonic acid is a key component in the biosynthesis of prostaglandins, as it is released from phospholipid molecules and converted by enzymes like cyclooxygenase and lipoxygenase to produce various prostaglandins and leukotrienes.
Prostaglandins also play a significant role in blood vessels, including vasodilation and vasoconstriction, which are crucial for cardiovascular function and regulation of blood pressure.
Endothelial cells are essential in the production and metabolism of various prostaglandins, including PGF2α, PGI2, and PGD2, contributing to cardiovascular homeostasis and vascular functions.
Initially, prostaglandins were celebrated for their potential in treating several medical conditions:
- Glaucoma: Reducing intraocular pressure to prevent vision loss.
- Labor induction: Used to stimulate contractions and induce labor.
- Gastrointestinal issues: Helped manage ulcers and other digestive problems.
Polyunsaturated fatty acids, such as arachidonic acid, are crucial in the synthesis of prostaglandins, serving as precursors for these bioactive lipid mediators.
Nonsteroidal anti-inflammatory drugs (NSAIDs) play a significant role in blocking prostaglandin synthesis, inhibiting the cyclooxygenase (COX) enzymes, and are clinically effective in treating pain, fever, and inflammation.
However, their powerful effects soon led to significant controversies and concerns. Some medical applications of prostaglandins resulted in severe side effects:
- Labor induction: Associated with excessive bleeding and uterine rupture.
- Eye treatments: Caused unexpected side effects like changes in eye color and vision issues.
These side effects raised ethical and safety questions. For instance:
- Patient safety: Were the benefits worth the risks?
- Informed consent: Did patients fully understand the potential side effects?
The physiological function of prostaglandins includes vasodilation, inhibition of blood clot formation, and generating and resolving the inflammatory response.
The process of prostaglandin production involves the biosynthesis and metabolism of various types of prostaglandins, which are crucial for inflammation and other physiological functions.
The controversies surrounding prostaglandins in medicine highlight the importance of rigorous safety evaluations. Introducing such potent substances into beauty products, as seen later, without thorough testing and transparency, poses significant risks.
Adoption in Cosmetics: Inflammatory Response Risks Overlooked
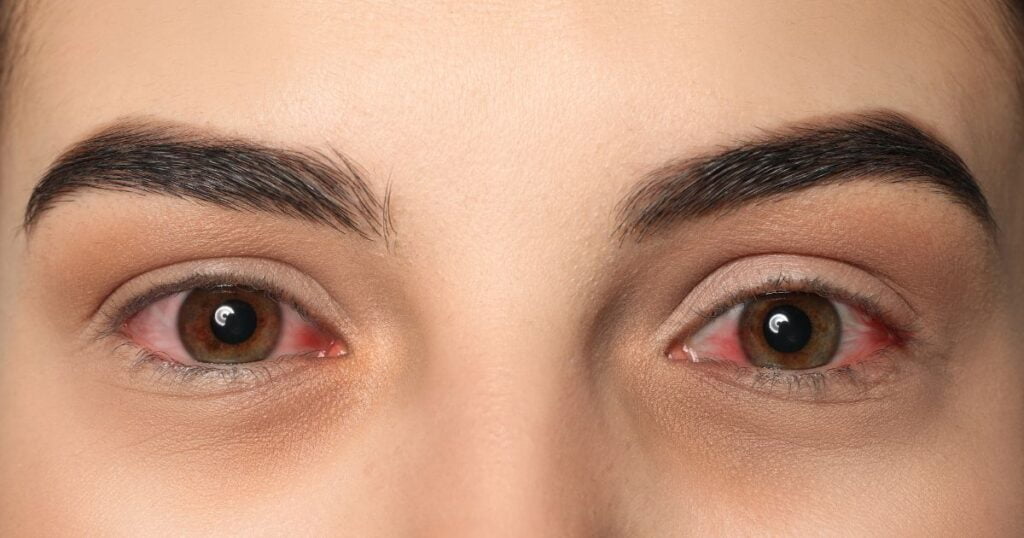
The cosmetic industry quickly saw the potential of prostaglandins for enhancing eyelash growth. Products like eyelash serums containing prostaglandin analogs promised longer, thicker lashes, leading to a surge in popularity. However, the rush to capitalize on this trend often meant overlooking significant risks.
Soon after their introduction, adverse reactions to prostaglandin-containing cosmetics began to surface. Common issues included:
- Eye irritation: Users reported redness, itching, and discomfort.
- Permanent eye color changes: Some users experienced darkening of the iris.
- Vision problems: There were cases of blurred vision and other eye-related side effects.
Despite these issues, early marketing efforts often downplayed or ignored these risks. Many consumers were unaware of the potential side effects associated with these products.
From a clean beauty perspective, this lack of comprehensive safety testing and transparency is troubling. Clean beauty principles prioritize:
- Safety: Ensuring products are free from harmful ingredients.
- Transparency: Providing clear and honest information about product contents.
The initial adoption of prostaglandins in cosmetics fell short of these standards. Companies prioritized profit and rapid market entry over consumer safety and full disclosure.
Regulatory Battles and Consumer Safety of Nonsteroidal Anti-Inflammatory Drugs
The use of prostaglandins in beauty products sparked significant regulatory battles. Agencies like the FDA faced challenges in classifying these substances. Were they drugs or cosmetics? This ambiguity created confusion and delays in setting safety standards.
Key regulatory issues included:
- Classification: Determining if prostaglandin-containing products should be regulated as drugs due to their potent biological effects.
- Safety endorsements: Delays in obtaining necessary safety approvals led to consumer uncertainty.
- Labeling requirements: Clear guidelines on product labels were often missing, leaving consumers uninformed about potential risks.
The lack of clear regulatory guidelines meant that many consumers were unaware of the possible side effects. Without stringent safety testing and transparent labeling, people using these products faced unforeseen health risks.
From a clean beauty standpoint, this situation highlights a significant conflict. Clean beauty emphasizes:
- Transparency: Full disclosure of all product ingredients and potential risks.
- Consumer safety: Ensuring products are safe for all users through rigorous testing.
The regulatory confusion around prostaglandins conflicted with these principles. For the clean beauty industry to maintain trust, clear and strict regulations are essential. This ensures that all products meet high safety standards and that consumers are fully informed.
Ethical Concerns and Clean Beauty Backlash of Physiologically Active Substances

The use of prostaglandins in cosmetics also raises significant ethical concerns. These issues go beyond health risks and jumps into the methods used to produce and test these compounds.
One major ethical issue is animal testing. The production and testing of prostaglandins often involve animal experiments, which many clean beauty advocates strongly oppose. The clean beauty movement prioritizes cruelty-free products, pushing for alternatives to animal testing.
Additionally, the environmental impact of sourcing prostaglandins cannot be ignored. The production process may involve harmful practices that damage ecosystems and contribute to pollution. Clean beauty champions sustainability and environmental responsibility, making the use of such compounds controversial.
Key ethical concerns include:
- Animal testing: Many prostaglandin-based products are tested on animals, conflicting with cruelty-free principles.
- Environmental harm: Sourcing and producing these compounds can negatively impact the environment.
- Transparency: There is often a lack of information provided to consumers about these ethical issues.
The backlash from clean beauty advocates has been significant. They demand:
- Ethical sourcing: Using methods that do not harm animals or the environment.
- Transparency: Clear information about the production and testing processes.
- Accountability: Holding companies responsible for ethical practices.
Brands that claim to uphold clean beauty standards while using prostaglandins face scrutiny. Consumers expect honesty and adherence to ethical principles. The ongoing criticism from clean beauty advocates highlights the need for companies to align their practices with the values they promote.
The Controversy of Prostaglandins

The history of prostaglandins in the cosmetics industry is fraught with controversy and challenges. From their medical origins to their contentious adoption in beauty products, these powerful compounds have raised significant health and ethical concerns. The clean beauty movement, with its emphasis on safety, transparency, and ethical practices, highlights the importance of scrutinizing such ingredients.
Key Takeaways:
- Health risks: The adoption of prostaglandins in cosmetics without thorough testing led to adverse reactions and consumer harm.
- Regulatory issues: Ambiguity in classification and delays in safety endorsements undermined consumer trust.
- Ethical dilemmas: Animal testing and environmental impact clashed with clean beauty principles, leading to significant backlash.
Reflecting on these issues underscores the need for stricter regulations and more rigorous testing. As consumers become more aware and demand higher standards, the beauty industry must prioritize safety and ethical considerations. By reevaluating the use of complex biological agents in beauty products, companies can better align with clean beauty values, ensuring a safer and more transparent future for all.

